Comprehensive up-to-date news coverage by Chemron FDA Korea.
IMPORTANT INFORMATION ON THE MEDICAL DEVICE USER FEE RATES FOR FY 2011
(October 1, 2010 through September 30, 2011)
Greetings Registered Establishment:
This letter is to notify you of the fiscal year 2011, (October 1, 2010-September 30, 2011), fee rates and payment procedures for medical device user fees payable to the United States Food and Drug Administration, (US FDA).
The Federal Food, Drug, and Cosmetic Act, (FD&C Act), as amended by the Medical Device User Fee Amendments of 2007, (“the 2007 Amendments”), authorizes the FDA to collect user fees for certain medical device applications. Fees apply to Premarket Approvals, (PMAs); Product Development Protocols, (PDPs); Premarket Reports, (PMRs); Biologics Licensing Applications, (BLAs for certain medical devices reviewed by FDA's Center for Biologics Evaluation and Research); some supplements; and Premarket Notifications, [510(k)s]. Additionally, the 2007 Amendments authorize the FDA to collect fees for 30-day notices; requests for information regarding classification [513(g)s]; and annual fees for periodic reporting on class III medical devices and for the registration of certain medical device establishments. The annual establishment registration fee must be paid between October 1, 2010 and December 31, 2010.
If you plan to send a submission to the FDA, payment must be received on or before the time you send it. If an applicant has not paid all fees owed, FDA will consider the application incomplete and will not accept it for filing or review. Small businesses may qualify for a waiver or a reduced fee on certain submissions to FDA.
FY 2011 Fees for Establishment Registration
The 2007 Amendments changed section 510(p) of the act to require electronic submission of registration and listing information. This includes both the initial registration and annual registration. You will list your medical devices at the same time, but there is no fee for device listing.
For assistance with registration and listing, please go to the CDRH website on Device Registration and Listing: (http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/RegistrationandListing/default.htm).
For fiscal year 2011 the registration fee for each establishment is $2,179 US Dollars.
There are no waivers or fee reductions for small business establishment registration. Every establishment pays the same fee.
FY 2011 Small Businesses; Fee Waiver and Fee Reduction regarding certain Medical Device Applications
In an effort to reduce the burden on small businesses, FDA provides a reduced rate for firms that meet the definition of a small business. The definition of a small business has not changed since 2006, i.e. $100 million or less in gross receipts or sales, including that of all affiliates. Small firms with gross receipts or sales of $30 million or less are eligible to have the fee on their first PMA waived. Both U.S. firms and firms based outside the U.S. may apply to FDA to qualify for a small business fee reduction. For further information about qualifying as a small business, please go the CDRH Website on Medical Device User Fee and Modernization Act at: (http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/MedicalDeviceUserFeeandModernizationActMDUFMA/default.htm) From the user fee page, please select “FY 2011 Medical Device Small Business Qualification and Certification” for instructions.
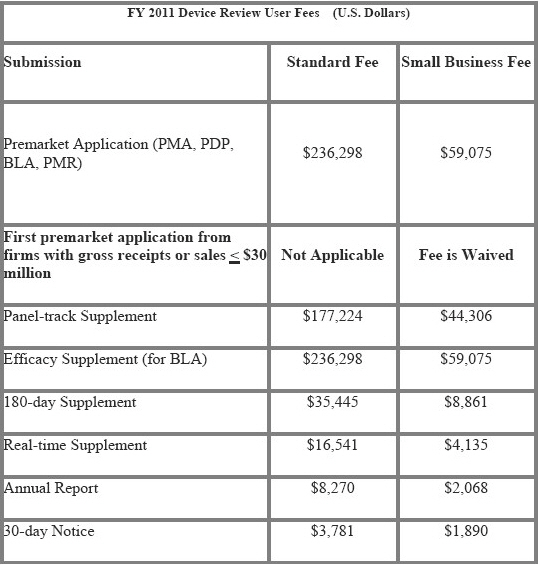
FY 2011 Application Fees
The FY2011 fees apply to applications received on or after October 1, 2010. If both the application and payment are received prior to October 1, 2010, you should pay the FY 2010 fee.
When submitting an application, do NOT send payment to FDA with your application. Instructions regarding how and where to send payment and how to qualify as a small business, are available on “MDUFMA Fee Page,” (http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/MedicalDeviceUserFeeandModernizationActMDUFMA/ucm109177.htm)
FY 2011 Fees for Review of Premarket Notification Submissions [510(k)s] and Requests for Information Regarding Classification [513(g)s] 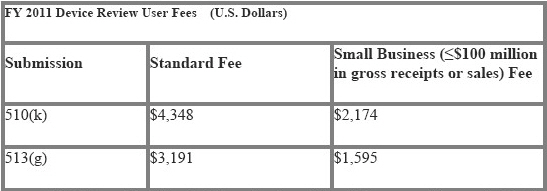
The Division of Small Manufacturers, International, and Consumer Assistance (DSMICA) can answer questions about user fees, regulatory requirements, and help you find guidance documents. DSMICA can be contacted by phone at 800-638-2041 or 301-796-7100 or by email at DSMICA@FDA.HHS.GOV. Questions regarding products regulated by the Center for Biologics Evaluation and Research (CBER) should be directed to the Office of Communication, Outreach and Development (OCOD), Manufacturers Assistance and Technical Training (MATT) Branch. CBER MATT can be contacted by phone at (800) 835-4709 or (301) 827-1800 or by email at MATT@FDA.HHS.GOV
Further information regarding medical device user fees is available at: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/MedicalDeviceUserFeeandModernizationActMDUFMA .